In September, our CEO, Raoul Lorenz, joined Steve Gens’ podcast to discuss the evolution of regulatory affairs and LORENZ’s pivotal role as a bridge between industry and agencies. Since the early 1990s, we’ve focused on delivering unmatched quality and expertise. They discussed several topics and questions like how LORENZ maintains a high standard or why even our CEO reads support tickets from time to time. Don’t miss this chance to get more detailed information on some really interesting and exciting topics they talked about. For the full conversation, click here.
A major highlight this year was our partnership with DNAnexus, enabling Trusted Regulatory Spaces in the cloud. This initiative, showcased through Project PRISM, supports collaborative validation and scientific review processes for regulatory submissions. Together, we’re working to:
- Facilitate seamless sponsor-regulator communication in secure, cloud-native environments.
- Enable real-time collaboration for drug and biologics submissions.
- Improve the efficiency of global regulatory processes, ensuring faster access to life-changing therapies.
This collaboration underscores our vision of leveraging cutting-edge technology to modernize regulatory practices worldwide.
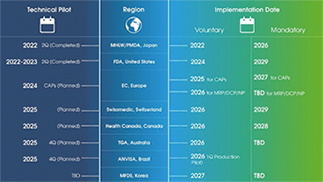
Simplifying eCTD 4.0 Transition
The global regulatory landscape is evolving, with eCTD 4.0 becoming the new standard. At LORENZ, we’re committed to making this transition smooth and stress-free for our customers. Explore our resources for practical insights and timelines
here.
From innovative partnerships to leadership in industry standards, 2024 has been a year of growth, collaboration, and impact for LORENZ. As we look ahead, we remain dedicated to empowering our customers and shaping the future of regulatory affairs.
Stay tuned for the last part of our blog series!
Click here to read the blog about Milestones & Memories (part1)
Click here to read the blog about Milestones & Memories (part2)
Click here to read the blog about Milestones & Memories (part4)