Please select a version:
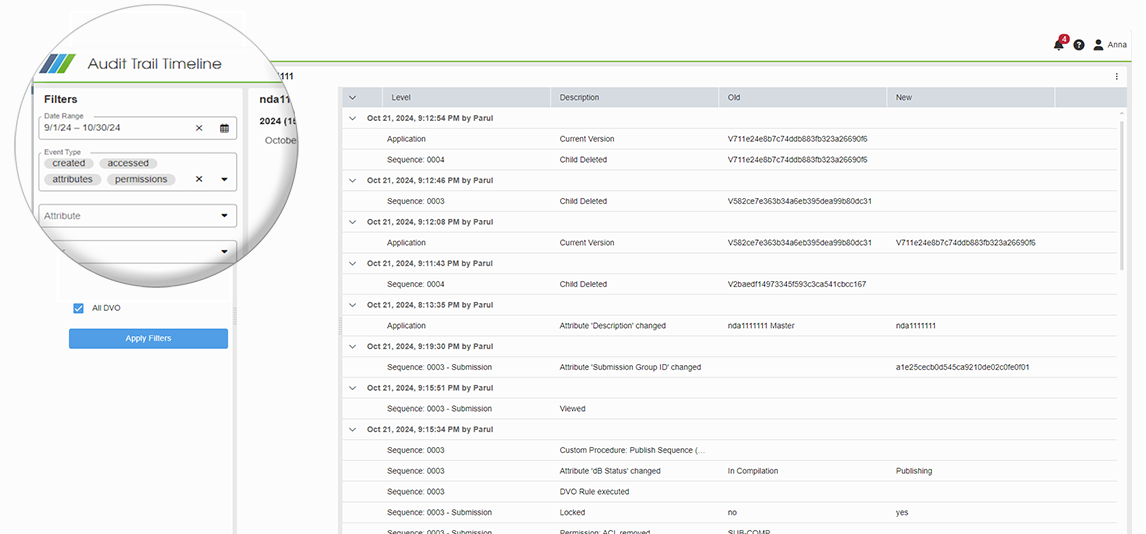
Forward Compatibility with eCTD 4.0
docuBridge now allows you to easily continue eCTD v3.2 dossiers in the new eCTD 4.0 version by simply changing the format, enabling forward compatibility. It automatically handles all conversions, and you can still update, replace, and merge documents between versions. This streamlines the process, making submission management more efficient and fully compatible with the new eCTD v4.0 format for future use.
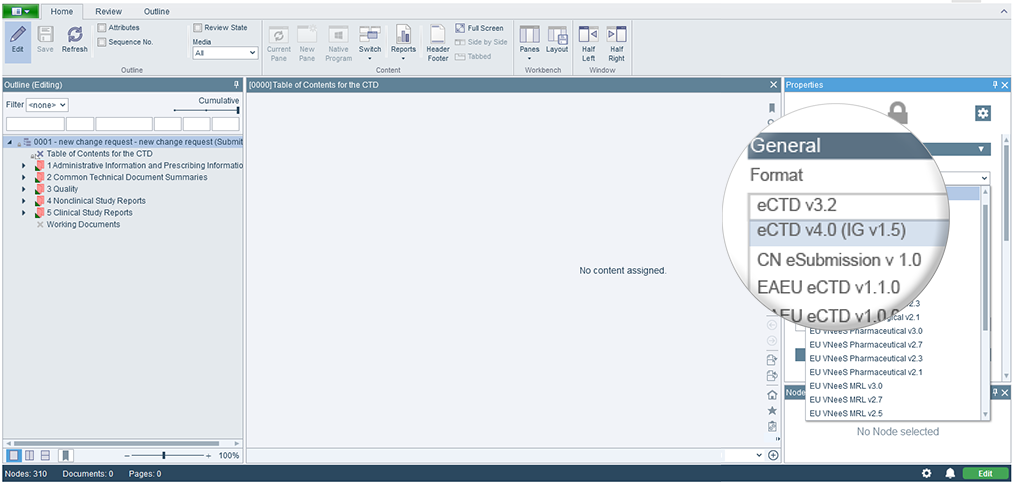
Enhancing the Submission Repository
The Submission Repository has introduced several enhancements in version 24.2. It now supports structured pools, offering more flexibility while scanning locations . A new location type, Packages, has also been added, enabling the management of various file and folder structures, including SPL data and even non-eCTD sequences. Users can efficiently scan configured package pools, search for package names, and apply filters to distinguish between different send statuses. Additionally, documents can be uploaded as attachments to sequences and packages, with a dedicated overview in the details pane. Finally, the sending workflow to regulatory authorities has been enhanced , now including templates for EMA ESG, EMA CESP, and the US FDA 'OC' Center, alongside the existing templates CBER, CDER, and HC.
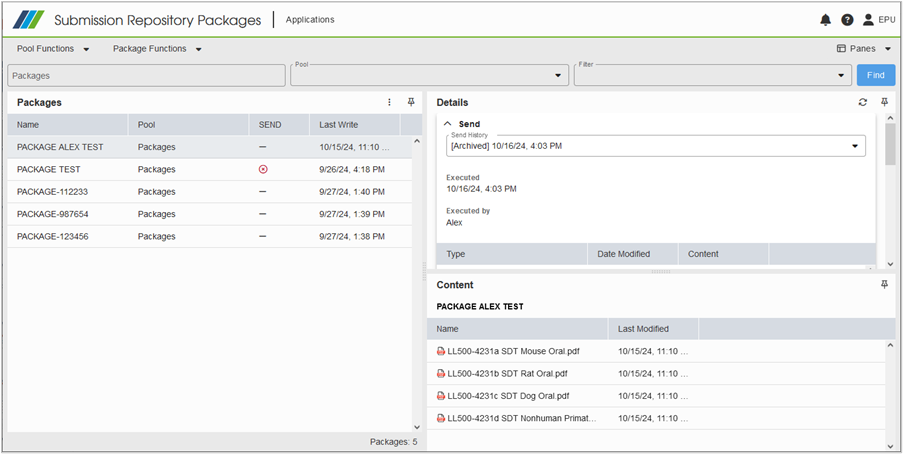
Document Audit Trail Timeline: A Clear View of All Changes
The new Audit Trail Timeline provides a comprehensive, timeline-based view of all changes to a selected content item—whether it’s a document, sequence, or search. With flexible filters, you can quickly narrow down results by date range, event type, attribute, or user, making it easy to find exactly what you need. Plus, for a deeper dive, you can display the full audit trail across the entire DVO structure of the chosen item.