LORENZ RIM
LORENZ provides a RIM solution designed to help you manage regulatory information in an easy and convenient way. It offers you capabilities to track, organize, and maintain regulatory information, including submissions, Health Authority correspondence, regulatory activities, and approvals from regulatory agencies such as the USFDA, EMA, and others. What makes our RIM solution unique is the flexibility it grants you by choosing even third-party capabilities to connect while still offering a seamless experience ‐ making it your own individual RIM solution.
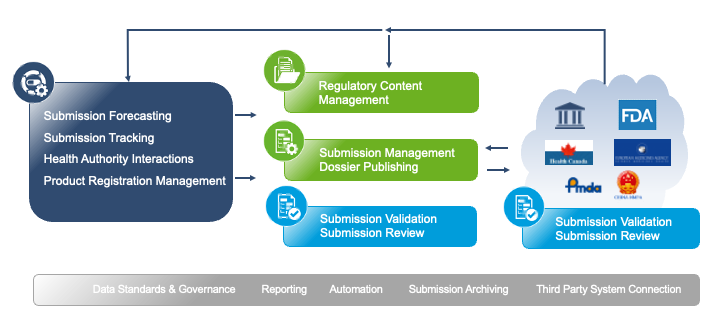
RIM and submission circle simplified
Benefits of the LORENZ RIM solution
Flexibility
Take full advantage of our RIM product suite, or pick and choose the capabilities you need. Our RIM solution offers the ultimate flexibility, even allowing you to seamlessly integrate with third-party solutions.
Intuitive user interface
Enjoy an effortless user experience designed to maximize usability and productivity.
Improved collaboration
Provide a single platform for working, sharing, and reviewing regulatory information to increase the collaboration between teams and departments.
Reduced risk of errors
Improve your data quality by having a single source of truth for your regulatory information. Automate the entry and propagation of regulatory data throughout your system, reducing the risks that come with manual or double data entry.
Simplified workflows
Streamline all regulatory tasks within our RIM platform instead of jumping from application to application.
Intelligent automation
Ensure compliance by using workflow automation to track submission requirements, regulatory activities, and more.
Easier maintenance
Reduce costs and maintenance through centralized platform administration and user management across our capabilities.
Access control customization
Tailor access permissions to meet your organization's unique needs, allowing you to control which users can view, edit, or manage regulatory information within the system.
Do you want to get a first look at how our RIM solution can provide you with a seamless experience?
Want to have a closer look at the single capabilities
Submission Management
Streamline your submission compilation to submit your applications easier, faster, and with confidence with our Submission Management solution.
Regulatory Content Management
Use intuitive workflows to review and approve documents, establishing a single source of truth for your regulatory content.
Submission Validation
Guarantee compliance with the latest global regulatory requirements for your electronic submissions. Our advanced technology ensures that your submissions meet all necessary standards, preventing technical errors and providing a smooth and hassle-free submission process.
Post-publishing
Enhance regulatory processes with a centralized post-publishing, optimizing efficiency and enabling easy global distribution from a central hub to global gateways and others.
Product Information Management
Simplify managing product information for regulatory compliance with our advanced solution. Easily submit xEVMPD data, prepare for IDMP, and track Health Authority Commitments and Interactions with streamlined reporting.
Lifecycle Management
Optimize your product's lifecycle management with our advanced solution for Regulatory Affairs. From ideation to post-market surveillance, our solution provides a seamless and efficient workflow for all stages of the product lifecycle, enabling you to make informed decisions based on real-time data.
Third-Party Integration
Enjoy the ultimate flexibility of our LORENZ RIM platform within your system landscape. Our technology is designed to work flawlessly to meet your specific business needs, whether that means taking advantage of our full RIM portfolio, or integrating our products with third-party systems.