Companies have joined with industry leaders and FDA on Project PRISM to advance solutions that can improve the submission, review and ease of communication for human drug and biologics applications
MOUNTAIN VIEW, Calif. & FRANKFURT, Germany — Feb. 12, 2024 — DNAnexus, Inc., provider of the Precision Health Data Cloud, and LORENZ Life Sciences Group, the leader in regulatory information management technology for the life sciences industry, today announced a partnership to offer Trusted Regulatory Spaces — secure, cloud-native environments that improve the submission validation process and ease of sponsor-reviewer communication for human drug and biologics applications to health authorities. The engagement is highlighted by Project PRISM, a research collaboration between Industry and the U.S. Food and Drug Administration (FDA) to demonstrate the feasibility of collaborative regulatory submission validation and scientific review utilizing LORENZ’s eValidator solution deployed on precisionFDA, FDA’s production cloud platform built and powered by DNAnexus for multi-omics and real-world data regulatory science and review. The joint offering leverages in-production solutions that already meet the most stringent security, privacy and data residency requirements and have been adopted by many leading global health authorities and life sciences organizations.
The regulatory submission process is challenging and resource-intensive for sponsors and health authorities. Digital transformation through the deployment of a trusted cloud platform that enables seamless collaboration between regulators and sponsors will accelerate the review and approval of life-changing therapies. To this end, DNAnexus and LORENZ are pioneering Trusted Regulatory Spaces to:
- Enable collaborative validation of regulatory submissions, allowing sponsors and health authority reviewers to leverage a secure cloud environment for eCTD validation and review
- Facilitate real-time communication between sponsors and regulators, for interactive review in collaborative Trusted Regulatory Spaces utilizing regulatory and scientific tools and technologies that enable enhanced sponsor-health authority interactions for the assessment of human drugs and biologics
- Provide sponsors and regulators with both private and shared workspaces, allowing them to maintain secure separation and expose only the data they need to, when they need to
The collaboration between both organizations will be on display during Project PRISM, which is being run in conjunction with FDA and industry collaborators, including two leading global pharmaceutical companies.
Built and powered by DNAnexus, precisionFDA launched in 2015 to enable the advancement of regulatory science and has evolved into a trusted, FedRAMP-authorized platform for secure collaboration and cloud computing. Since its inception, precisionFDA has grown to support collaborative use cases around genomics, multi-omics, real-world data, and AI and machine learning. Project PRISM represents the next adaptation of precisionFDA and seeks to help life sciences organizations and global health authorities leverage regulatory and scientific cloud platforms to achieve greater efficiencies on a regional and international scale. precisionFDA has a FISMA Authorization to Operate, building on DNAnexus’ FedRAMP authorization and 21 CFR Part 11 Computer System Validation, and platform features, such as chain of provenance tracking and data immutability, to deliver a regulatory-grade service for collaborative science in the cloud.
LORENZ has been a leader in regulatory technology since 1989 and provides mission critical electronic application review and validation systems to 17 global regulatory authorities across global regions such as North America, Europe and Asia-Pacific. The company is playing a key role enabling technology to support a modernized trans-regional collaborative joint review process for approval of innovative medicines through the ACCESS Consortium NASWSI - New Active Substance Work-Sharing Initiative. Today, 27% of the world’s population is receiving new medicines approved electronically through LORENZ solutions. As part of the start of this collaboration, LORENZ’s off-the-shelf eValidator solution will be leveraged within the secure confines of precisionFDA, enabling sponsors to share eCTD validation results with reviewers using shared interactive workspaces called Trusted Regulatory Spaces.
“The regulatory submission and review process is the perfect opportunity to showcase the secure collaboration capabilities of the DNAnexus platform,” said Thomas Laur, CEO of DNAnexus. “We are excited to work with LORENZ to deliver a trusted cloud solution that is ready to be adopted by global regulators and sponsors today.”
“We are honored to be working with DNAnexus to enable a future where health authorities can collaborate with each other and sponsors can securely process applicant/regulator interactions with various health authorities in all regions, on regulatory submissions in the cloud,” said Wolfgang Witzel, President of LORENZ Life Sciences Group.
Both organizations will be available to discuss Trusted Regulatory Spaces at the upcoming DIA RSIDM conference, taking place February 12-14 in Washington, DC.

About DNAnexus
DNAnexus enables biomedical organizations to accelerate scientific discovery and improve patient care with the Precision Health Data Cloud. The company provides scientific innovators and healthcare professionals with the ability to manage, analyze, and collaborate on multi-omic, clinical and real-world data to unlock insights. DNAnexus actively manages more than 105 petabytes of data on behalf of a growing network of collaborations with leading pharmaceutical, clinical diagnostic, academic research, biobank, and government organizations. Today, more than 45,000 users across 48 countries and over 130 enterprise customers are harnessing the full potential of their data with the scalable and secure Precision Health Data Cloud. DNAnexus is headquartered in Mountain View, Calif. For more information, visit www.dnanexus.com or follow @DNAnexus.
About LORENZ
LORENZ Life Sciences Group (http://www.lorenz.cc/) has been developing and marketing software solutions for the Life Sciences market for over 30 years. The LORENZ Regulatory Information Management solutions address industry, health authorities and academia to ensure compliance enforcement worldwide. LORENZ's proven portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third-party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. LORENZ has a strong customer base worldwide, with over 1800 paid installations in 48 countries, including 17 agencies.
Frankfurt/Main, Germany and Singapore, Singapore, June 7, 2023 // The consortium of LORENZ Life Sciences Group, VRG Pte. Ltd. & WebSparks Pte. Ltd. has been awarded the contract to provide an eCTD reviewing solution to the Singapore Health Sciences Authority (HSA). This solution will receive and review eCTD dossiers and assist in the development of a regional schema and eCTD specification.
The consortium will provide a complete eCTD solution, comprised of LORENZ’s core software applications: docuBridge, eValidator and Automator; as well as a portal offering from WebSparks. Project management, consulting, training services and support will also be provided by all consortium members.
HSA intends to introduce the eCTD system gradually and its adoption for dossier submissions will be optional. The system's initial release will cater to new drug applications, generic drug applications, and their corresponding Drug Master File (DMF) submissions.
“We are honored to be part of the consortium which will support Singapore’s efforts to optimize its drug evaluation and approval system. We are confident that together, we can provide an integrated, world-class solution that will fully address their needs,” says Wolfgang Witzel, President of LORENZ Life Sciences Group.
LORENZ is committed to working closely with HSA to ensure a successful implementation of the eCTD system. This partnership marks another milestone in LORENZ's continued mission to support global regulatory authorities in adopting eCTD technology.
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc ) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ offers a wide array of Regulatory Information Management solutions geared towards industry, health authorities and academia in order to support compliance globally. LORENZ's tried and tested solutions cover product registration/IDMP, submission assembly, validation and management, publishing/eCTD, regulatory planning and tracking products, as well as related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow its customers to enhance their operational efficiencies. With over 1700 paid installations in 48 countries, LORENZ has a strong worldwide customer base. The company headquarters is located in Frankfurt, Germany.
To date, LORENZ has established eCTD systems for 15 national health authorities, including the USFDA (USA), EDQM (Europe), BfArM (Germany), Health Canada (Canada), TGA (Australia), HPRA (Ireland), Thai FDA (Thailand) and CDE/NMPA (China).
Frankfurt/Main, Germany and Silver Spring, MD, November 2, 2020 // The U.S. Food and Drug Administration (USFDA) is now using LORENZ docuBridge and eValidator. The Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER), are both using the LORENZ solutions now to process incoming as well as legacy submissions. The eValidator validates all incoming submissions for technical compliance, and docuBridge is used to review submission content in the context of the entire life cycle of an application.
This ‘go-live’ concludes a lengthy process that began in 2016, when LORENZ won the FDA's first tender for a submission review solution, and won again in 2017 when a second tender was issued in response to protests following the first.
During the first stage of the project all 1.2 million legacy sequences were migrated into docuBridge, with all incoming sequences thereafter being imported on a regular basis, adding up to 1.5 million sequences. The migration took only 16 days. This year, amidst the challenging COVID-19 pandemic, over 4,000 reviewers at the CDER and CBER were trained virtually on how to use the LORENZ solutions for maximum efficiency in their everyday validation and review processes.
“We are deeply gratified that the USFDA has placed their trust in us,” said Wolfgang Witzel, President of LORENZ Life Sciences Group, “and we are sure we have delivered a range of high-quality solutions that will allow the CDER and CBER to validate and review submissions much more efficiently. We are happy to welcome the USFDA to the LORENZ ecosystem.”
With the go-live of docuBridge at the USFDA all companies using LORENZ docuBridge for their submission management needs now have a seamless submission process from assembling, approving, publishing and submitting via the gateway to the reviewers using docuBridge. For companies not using docuBridge, LORENZ offers a review only package to leverage the three key advantages of visibility, collaboration and peace of mind. This package is limited to reviewing functionality, and can be upgraded to a full submission management solution. Read more about it here.
“Now that this important milestone has been achieved, the LORENZ and USFDA teams look forward to closer collaboration, specifically in the area of eCTD 4.0,” said Yaprak Eisinger, Managing Director, North America of LORENZ Life Sciences Group. For more information on LORENZ's RIM portfolio, please visit LORENZ's website.
For more information on this project please contact LORENZ.
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc ) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ has an array of Regulatory Information Management solutions geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ's tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. With over 1300 paid installations in 38 countries, LORENZ has a strong worldwide customer base.
Frankfurt/Main, January 31, 2020 // LORENZlink, LORENZ’s North American Regulatory Information Management Solutions Conference will take place during April 21-22, 2020 in Vancouver, Canada. While the first 2 days of the conference will feature presentations and tutorials held by a distinguished faculty of LORENZ customers, partners and employees, participants will also have the option to remain for an additional day of hands on docuBridge training.
LORENZlink’s philosophy is to Converge, Connect and Collaborate, and as such many LORENZ customers and partners will share their own practical experiences and best practices relating to current hot topics, trends, products and services in regulatory affairs.
"We are so excited about the quality of abstracts we received this year. Our customer base and valuable partners will enable us to offer presentations and tutorials on strategic topics such as how the concept RIM or the role of a Chief Data Officer are evolving, and also address every day challenges on the minds of regulatory professionals – from technical rejection criteria for study data to achieving RA efficiency through process optimization. As always, there will be global regulatory agency updates from regions such as the US and China.” said Yaprak Eisinger, Managing Director for North America, adding that "We look forward to learning from each other while enjoying Vancouver’s spectacular views and multicultural, sustainably sourced cuisine.”

UPDATE from 5 March 2020: LORENZ regretfully canceled LORENZlink 2020.
If you have questions related to LORENZlink 2020 please contact us.
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc ) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ has an array of Regulatory Information Management solutions geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ's tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. With over 1050 paid installations in 38 countries, LORENZ has a strong worldwide customer base.
Frankfurt/Main, October 28, 2019 // LORENZ is proud to announce that it will be launching a new pre-configured LORENZ docuBridge FIVE package, limited to reviewing functionality and applicable for all regions currently accepting eCTD. The review package is easily upgradeable to a full Submission Management Solution and/or end-to-end RIM solution for those customers looking to expand capabilities.
It comes with three immediate benefits: Visibility, Collaboration, and Peace of Mind.
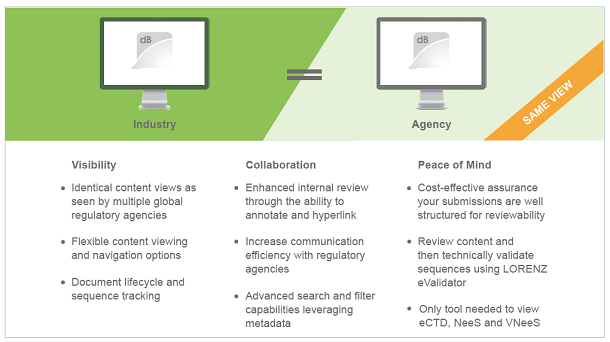
The solution is based on LORENZ’s collaboration with a myriad of global regulatory agencies and is an affordable option for reviewing submissions for any company submitting to USFDA, Health Canada, and many other global regulatory agencies. As a reminder, FDA’s CDER and CBER programs will use LORENZ eValidator and docuBridge to validate and review incoming and legacy eCTD submissions starting in early 2020, and the CDE of the Chinese NMPA will also implement both products as their eCTD Data Management System soon.
"The docuBridge FIVE review package offers companies a cost-effective and simplified option to review submissions easily in an increasingly complex regulatory environment,” says Hilary Hafeken, Regulatory Solutions Consultant at LORENZ and previously Director of Regulatory Operations with over 20 years of experience.
Please contact LORENZ for more information on this offering.
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc ) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ has an array of Regulatory Information Management solutions geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ's tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. With over 1050 paid installations in 38 countries, LORENZ has a strong worldwide customer base.
Frankfurt/Main, October 9, 2019 // More than 180 delegates from 25 countries attended the fully sold-out userBridge.19 conference from September 17-19, 2019 at the Divani Apollon Palace Hotel in Athens. This annual gathering hosted by the LORENZ Life Sciences Group has become the world’s leading regulatory affairs forum, drawing high-powered speakers and participants from industry, health authorities, and consultants.
Echoing the worldwide move toward electronic submissions, userBridge covered all current hot topics in Regulatory Affairs, including the transition from unstructured to structured data, the range of emerging regulatory standards, processes and optimization, as well as detailed regional updates on e-submissions in Russia and EAEU member states, southern Africa, China, Canada, and the UK.
CEO Raoul Lorenz opened the conference with a keynote address highlighting the Alliance of Experts, a partnership that can provide industry with a seamless end-to-end RIM experience. He also gave an update on agency projects LORENZ is currently handling at the US FDA and at China’s NMPA. He was followed by Christian Kaas, Managing Director of Research & Development at LORENZ, who gave an overview of recent and upcoming product refinements, including the updating of regulatory specifications without the need to reinstall the software.
Throughout the two-and-half day conference, a range of prominent speakers gave userBridge participants useful behind-the-scenes insights, including Dr. Frank Hoffmann-Geim from Schott, who shared his company’s decision process for cloud or on-premises implementation of docuBridge. The agency perspective was also covered by a variety of experts including Dr. Klaus Menges, Senior Regulatory Expert at the German BfArM.
Several userBridge speakers considered the digital future in regulatory affairs using NLP and voice assistant functions, including Susant Mallick and Steven Ford from Amazon Web Services. Christian Kaas presented LORENZ´ own regulatory assistant, Adam, and demonstrated how chat or voice can be used to create a new sequence for a running regulatory activity. In addition, Luka Kovacic of Novartis and Jason Berning of LORENZ outlined how the LORENZ Automator and ADAM benefit operations at Sandoz, and how its feedback helps improve both products.
In addition to the in-depth plenary presentations, userBridge once again offered its popular selection of table tutorials. These 32 mini-sessions were split into four rounds to give participants the chance to explore specific topics in a small group setting, and to select the topics most relevant to their own day-to-day work. Participants were also able to schedule meet-to-talk sessions with LORENZ team members to discuss important issues face-to-face.
The daytime agenda was accompanied by evening events with a relaxed, family-like atmosphere for networking and wide-ranging discussions. The “userBridge Band”, a group of conference guests and LORENZ staff, even provided musical entertainment.
“Once again userBridge covered the most important issues in the e-Regulatory Affairs world,” says CEO Raoul Lorenz, “and we’d like to thank all of the presenters and guests who worked so hard to make this year’s conference both memorable and relevant. We hope to welcome everyone to the next userBridge conference in Frankfurt in September 2020, or to LORENZlink in Vancouver in April 2020.”

About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc ) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ has an array of Regulatory Information Management solutions geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ' tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. With over 1050 paid installations in 38 countries, LORENZ has a strong worldwide customer base.
Frankfurt/Main, June 14, 2019 // The userBridge regulatory affairs conference hosted by LORENZ Life Sciences Group will take place in Athens, Greece from 17-19 September 2019. This annual gathering attracts and showcases the latest thinking in regulatory affairs, offering a range of perspectives from agencies, industry and prominent consultants. The event is also structured to allow generous time for one-on-one networking. Many participants return to this forum year after year, and newcomers always feel warmly welcomed.
This year’s conference venue will be the Divani Apollon Palace & Thalasso, one of Athens’ most iconic hotels. With the booking code 11195, userBridge participants can take advantage of a special room rate at the conference location, avoiding a daily trek to the event through the city’s heavy rush hour traffic.

Spread over 2½ days, userBridge offers its delegates both in-depth learning and the chance to exchange views with other regulatory affairs experts. In-depth plenary presentations are complemented by a wide range of other learning formats in small groups as well as on a one-on-one basis:
• LORENZ ‘Meet to Talk’ gives people the chance to book a private 45-minute slot with one or more of the company’s senior
executives.
• Table Tutorial sessions allow the detailed exploration of specific topics in small groups.
• The Agency Round Table enables participants to interact directly with agency representatives.
The conference agenda will cover a cross-section of current regulatory affairs issues, including:
• Agency updates from Health Canada, US FDA and South African SAHPRA
• Regional eSubmission updates on China, EAEU and GCC
• DMF eCTD for US and Canada
• Industry experience on automating submission process
• Merging of standards - eCTD, IDMP and structured product data
• Practical experience in preparing CP/MRP/DCP dossiers for Brexit
• Latest developments in African Medicines Regulatory Harmonization
• What's new in LORENZ products?
• Best practice advice on processes, content and software
"The simultaneous profusion and merging of standards and the increasing ability to create seamless workflows between multiple regulatory systems is providing a huge opportunity in our sector,” notes Raoul Lorenz, CEO of the LORENZ Life Sciences Group, “and we’re proud that userBridge has become an important gathering for anyone who wants to keep up with the latest developments in regulatory affairs. We hope very much to see you in Athens this September.”
To register for LORENZ userBridge.19 on 17-19 September 2019 in Athens please click here!
To take advantage of the userBridge special room rate at the Divani Apollon Palace & Thalasso, use the booking code 11195 and follow this link!
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc ) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ has an array of Regulatory Information Management solutions geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ' tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. With over 1050 paid installations in 38 countries, LORENZ has a strong worldwide customer base.
Frankfurt/Main and Beijing, June 12, 2019 // LORENZ and ERIS recently co-hosted the successful “2019 China eCTD Management and Application Workshop” in Beijing. The event was designed to assist pharmaceutical companies in structuring their submission management processes to meet China’s upcoming eCTD requirements. Participants received comprehensive training in the relevant eCTD submission management regulations and expected practices for the Chinese market.
“We were extremely proud to have experts from the eCTD project team at CDE, NMPA on hand to meet our participants and to share the latest progress on the Chinese eCTD projects, including the technical specifications and verification standards,” says Jeremy Yi, CEO of ERIS, “and we also covered eCTD basics and current global trends.”
In addition to health authority participants, a range of invited industry representatives shared their valuable practical experiences in creating submission-ready documents and compiling eCTD submissions, as well as their insights into creating the necessary processes and teams, the use of related software, and many other aspects.
Experienced trainers from both China and Germany took workshop participants through the entire submission process including compiling, reviewing, and validating an eCTD submission, as well as handling the lifecycle management, all demonstrated while using the LORENZ docuBridge FIVE solution. This is the software that the CDE has chosen for their own eCTD validation, review and management.
“It was gratifying to see how well all participants interacted with our eCTD experts,” says Frank Schroer, Senior Trainer at LORENZ, “and we believe that thanks to these two days of training, the participating companies are now much better prepared to meet the eCTD submission requirements in China.”

The CDE issued the Chinese eCTD specification and validation criteria draft and related documents for public comments on March 1, 2019 (click here). In addition, the NMPA published the Chinese M1 requirements and a translated version of the CTD on April 17, 2019 (click here). Together, these mark an important milestone in China´s eCTD project, kicking off the final countdown to the implementation of eCTD submissions in China.
According to the experts at the workshop, the Chinese eCTD specifications and verification standards have now been closed for comments. The eCTD submission guidelines and implementation announcements are currently being drafted and will soon be ready for public comments.
From a corporate perspective, introducing eCTD submissions is a massive undertaking; much more than just a short-term task. It means setting up an eCTD submission management system, including adapting management processes to it, and establishing cooperation between the drug development and drug registration teams, as well as building up and training the eCTD submission management team and understanding the actual eCTD regulations. Each of these aspects will directly impact the efficiency of a company’s eCTD submission management, and influence how quickly a drug will be approved for marketing.
“With the help of our experts and trainers, participants have obtained a good understanding of these important interrelationships,” notes Fabian Witzel, Corporate Development Manager at LORENZ, “and at the same time, this workshop helped us to gain a better understanding of the needs of the Chinese industry. We are truly proud to be a part of the Chinese eCTD project, supporting both industry players and the agency in taking this important step forward.”
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc) has been developing and marketing software solutions for the Life Sciences market since 1989. As a leading supplier of submission management systems for eCTDs, the solutions of LORENZ are geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ' tried and tested solutions specifically cover submission assembly, review, publishing, validation and management. Furthermore, the company’s portfolio covers product registration/IDMP, regulatory planning and tracking products, as well as related services.
To date, LORENZ has established eCTD systems for 12 national health authorities, including the US FDA (USA), EDQM (Europe), BfArM (Germany), Health Canada (Canada), TGA (Australia), and the Thai FDA (Thailand). It is currently assisting CDE, NMPA (China) in establishing its eCTD data management system.
About ERIS
ERIS (www.eris-bj.com) focuses on electronic regulatory services. Based on the experience of providing registration regulatory and information services, ERIS is dedicated to providing the clients with the world's most desirable e-regulatory affairs solutions, as well as local installation, configuration, training, regulatory consultation, and post maintenance services, fully meeting clients’ needs in eCTD application management.
Frankfurt/Main, April 23, 2019 // New Orleans played host to this year’s LORENZlink, LORENZ' Regulatory Information Management Solutions Conference, which took place on April 9 and 10, 2019. The event, attended by industry and health authority experts, featured presentations and tutorials geared to answer key questions encountered in today’s RIM landscape.
A joint Keynote by Raoul Lorenz, Christian Kaas and Yaprak Eisinger from LORENZ was followed by presentations from leading regulatory professionals working at Accenture, Bayer, Biogen, Gilead, Novartis, Pharmatech Associates, QDossier and Whitsell Innovations. Industry expert Steve Gens shared key trends and predictions on RIM 2022, while Health Canada’s Irena Pastorekova gave an update on eCTD. The event also offered optional training opportunities in creating eCTDs as well as the basics of how the FDA reviews applications for those who opted to extend their stay.

There was also time carved out for networking and fun! Participants were part of a second line parade through the streets of the French Quarter bubbling with local culture as they made their way to dinner on the first evening. Other venues with jazz and blues music provided unique backdrops for LORENZ customers and partners to continue to share ideas and insights after hours.
“We look forward to the privilege of hosting LORENZlink in Vancouver next April,” said Yaprak Eisinger, Managing Director, North America for LORENZ. “Spotless execution of education, coupled with thoughtful and fun activities,” “…hit it out of the ballpark with LORENZlink 2019,” were some of many rewarding pieces of feedback that LORENZ will continue to strive after in coming years.
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc ) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ has an array of Regulatory Information Management solutions geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ' tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. With over 1050 paid installations in 38 countries, LORENZ has a strong worldwide customer base.
Frankfurt/Main, Germany and Amersham, England and Sarasota, FL, February 12, 2019 // Generis, Phlexglobal and LORENZ Life Sciences Group, announce the founding of the Alliance of Experts, a new source for end-to-end Regulatory Information Management (RIM) and Clinical solutions in Life Sciences. The Alliance of Experts brings the expertise of each company together to provide seamless interoperability between their software packages, providing customers a ‘single source of truth’ for all data that can be used and applied in multiple solutions.
Prior to the founding of the Alliance of Experts, life science customers either employed a range of specialized tools from several different domain specialists to meet RIM requirements which may not work together or purchased a single solution RIM enterprise tool that provided limited depth of expertise in the rapidly changing life science market. The Alliance of Experts offers the best of both worlds by providing expert, established applications and guaranteeing the integrations between them with a single Alliance support service. This approach removes the burden of maintenance and risk from our customers, while ensuring zero compromise on functionality.
A prime example of an effective RIM solution provided by the Alliance of Experts is the Submission Archiving function. Leveraging the integration between LORENZ docuBridge and Generis’ CARA, Submission Archiving pushes an archive copy of the published submission into CARA for long term storage, while creating an application sequence in docuBridge that includes leaf nodes pointing to the relevant files in CARA. Archived submissions can be accessed from CARA or from docuBridge with equal ease, giving customers exactly the same view of the submission that the agency has, along with links to the source files of their published equivalents.
“The whole point of the Alliance of Experts is efficiency,” says James Kelleher, CEO at Generis, “and this means that customers benefit directly from having a single source of regulatory data that can feed many different processes. This means that the sheer number of sources of error are greatly reduced.”
Karen Roy, Chief Strategy Officer from Phlexglobal echoes the sentiment: “Combining our Clinical expertise with the Alliance’s Regulatory Affairs expertise really creates a whole new user experience. Customers can assemble their own perfect solution from a range of different partial ones.”
"The Alliance of Experts combines the advantages of a single RIM vendor and at the same time overcomes the limitations of a single RIM vendor," says Raoul Lorenz, CEO of LORENZ Life Sciences Group, "Through 'automated integration' we are taking the headache out of system integration, and are able to offer more variety and much deeper functionality. This is a marked step forward from both the classic approaches as well as the rigid platforms that have tried to replace them."
For more information of how the Alliance of Experts has created agile, tailored, end-to-end solutions to help customers, check out the joint initiative’s website.
About LORENZ
LORENZ Life Sciences Group (www.lorenz.cc) has been developing and marketing software solutions for the Life Sciences market since 1989. LORENZ has an array of Regulatory Information Management solutions geared towards industry, health authorities and academia that enable compliance enforcement globally. LORENZ' tried and tested portfolio offers Product Registration/IDMP, Submission Assembly, Validation and Management, Publishing/eCTD, Regulatory Planning and Tracking products and related services. Interoperability between LORENZ products and third party solutions, as well as the ability to automate processes, allow LORENZ customers to enhance operational efficiencies. With over 1050 paid installations in 38 countries, LORENZ has a strong worldwide customer base.
About Generis Knowledge Management
Generis has been providing leading-edge software for content management systems including Documentum, SharePoint, Alfresco and Oracle WebCenter since 1997. The company has more than 50 customers across industries from Federal Government, through Life Sciences and Engineering to Media, Publishing and Financial. The company is headquartered in Sarasota, FL, and has its principal development centers in Europe. Customers include 8 of the global top 10 Life Science companies, Federal and State governments, engineering and media companies. For more information about Generis visit www.generiscorp.com.
About Phlexglobal
Phlexglobal are world renowned thought leaders and specialists in the provision of electronic Trial Master File (eTMF) systems and services. They offer a unique combination of eTMF technology with PhlexEview – their market leading eTMF system, quality services and specialist resource that delivers a range of flexible, targeted solutions to meet your business needs. Phlexglobal is headquartered in the UK, with offices in Malvern, PA and Lublin, Poland.