Status of ISO IDMP and what DADI has to do with it
Posted on March 10, 2022
Since the EMA has changed the ISO IDMP timeline in order to push the Digital Application Dataset Integration Project (DADI), we want to offer you an overview of what has changed and the reasons behind it.
Why the ISO IDMP timeline has changed
EMA has announced changes to the EU IDMP implementation approach and postponed the implementation of FHIR messages, which have been moved from Q1 2022 (optional submissions for the Centralised Procedure) to a later date. This decision was made to bring the Digital Application Dataset Integration Project (DADI) forward. One of the reasons why this has been decided is that seven NCAs, including AGES and BfArM, have obtained funding to implement ISO IDMP compatible electronic application forms (eAF). Their goal is to accelerate ISO IDMP implementation in Europe. Another, more obvious reason is that current Adobe PDF forms are aging and risk no longer being fit for purpose.
There is also a long-standing need to improve electronic application forms to support efficiency and interoperability and the current application form tools nearing end of life.
What is DADI?
The Digital Application Dataset Integration Project (DADI) will replace current PDF-based electronic application forms with new web-forms on an updated portal. It is going to enable both the familiar human-readable (PDF) output and a new machine-readable output for digital processing, using the FHIR data exchange standard for medicinal products. In order for DADI to comply with FHIR, the release of the final FHIR specifications is still planned for Q2 2022 as seen below in Figure 1. In addition, in order to enable both outputs, DADI will structure the input of electronic application forms to capture standard product data (PMS), thus ensuring ISO IDMP compliance. The data from the new web-based eAF will feed and continue to enrich the PMS data, which at this initial stage will be based on existing XEVMPD and SIAMED submitted product data. For variations applications for human medicinal products, the forms will pull available PMS data to prepopulate applications where relevant, thus ensuring data consistency. The DADI project will therefore enhance regulatory process efficiency through an interconnected network for the management and assessment of submissions and the increased use of existing and consistent set of structured data for all regulatory procedures in the future.
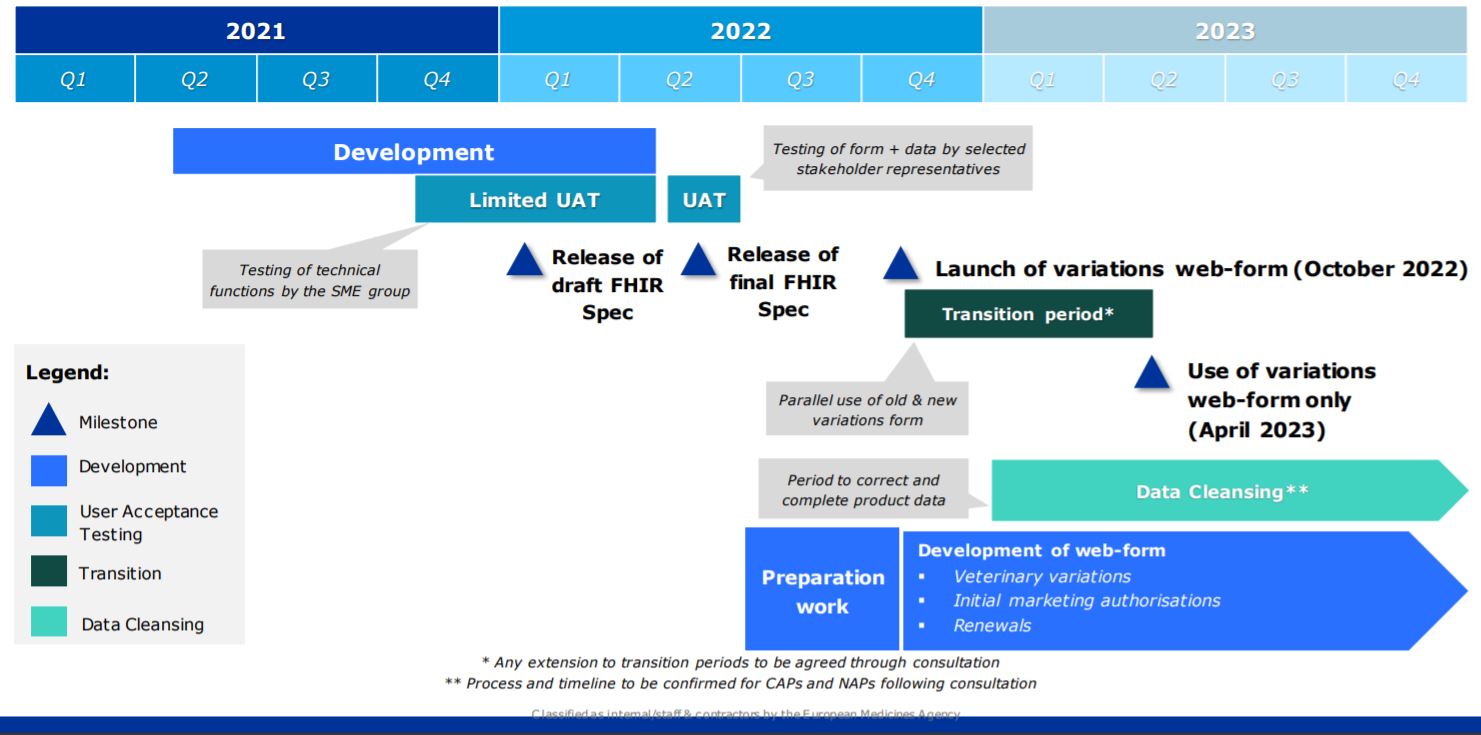
Figure 1: EMA Website “DADI Human Variations Form Timeline” (February 2022)
EMA’s long-term plan
The European Medicines Agency wants to move from core regulatory business optimization to regulatory digital business transformation, with regulatory business optimization being and continuing to be the foundation for EMA’s digital transformation. To lay this groundwork, they first want to harmonize regulatory processes, enable and improve processes digitally, and increase reliability and usability of structured data. In order to meet this goal, EMA aims to create a single, high-quality source of data to use and exchange across processes and systems inside and outside the agency – especially product and substances master data, i.e., Product Management Service (PMS) and the Substance Management Service (SMS).
Timeline DADI
At the moment, DADI is planned for all variations procedures for human medicines (centralized and national, including DCP/MRPs). This will be followed by initial marketing authorizations and renewals (human only). Other submissions procedures (Veterinary and MA Transfer) are also planned for later. EMA plans the launch of variations web-form for October 2022 which will be followed by a 6-month transition period where both the PDF eAF and the web-based form can be used simultaneously. As of April 2023, only the web-based form will be usable.
What does this mean for IDMP?
Although an updated roadmap for IDMP hasn’t been released yet, it is safe to say that with the postponed timeline for FHIR messages for CAPs, the date for the planned implementation of IDMP in the EU will most likely be changed as well. Many details on the new timeline and the updated plan are not yet available, but we can be assured that EMA is currently working on it by focusing on “DADI first for all product types, FHIR message later.” What we can already say is that IDMP is still a relevant topic and a current driver in the regulatory affairs world as it is going to become effective sooner rather than later, especially if the DADI project is able to accelerate the ISO IDMP implementation in Europe.
How LORENZ can help you with the upcoming IDMP challenge
LORENZ drugTrack can help you to prepare for IDMP by identifying and understanding compliance gaps and allowing you to already implement IDMP data into the system. If you want to find out more on how drugTrack can be of help in the upcoming IDMP challenge, click here.
This entry was posted in Regulatory Affairs News.

