The 25.1 releases of docuBridge, drugTrack, and eValidator are available!
Posted on April 30, 2025
Release Highlights:

What's new in docuBridge? The advanced Node Content Pane improves efficiency in complex eCTD 4.0 applications with faster performance, clearer replace overviews, and customizable document views. The new webAccess App lets you use docuBridge in a distraction-free environment on Chrome or Edge, with all the same features as the browser version. The webAccess Explorer now includes Search Management, so authorized users can create, edit, share, and manage searches and application views with ease. Discover more about these features here: What's new in docuBridge 25.1?
This entry was posted in LORENZ Solutions and Services.
Sneak peek at our upcoming 25.1 releases
Posted on April 25, 2025
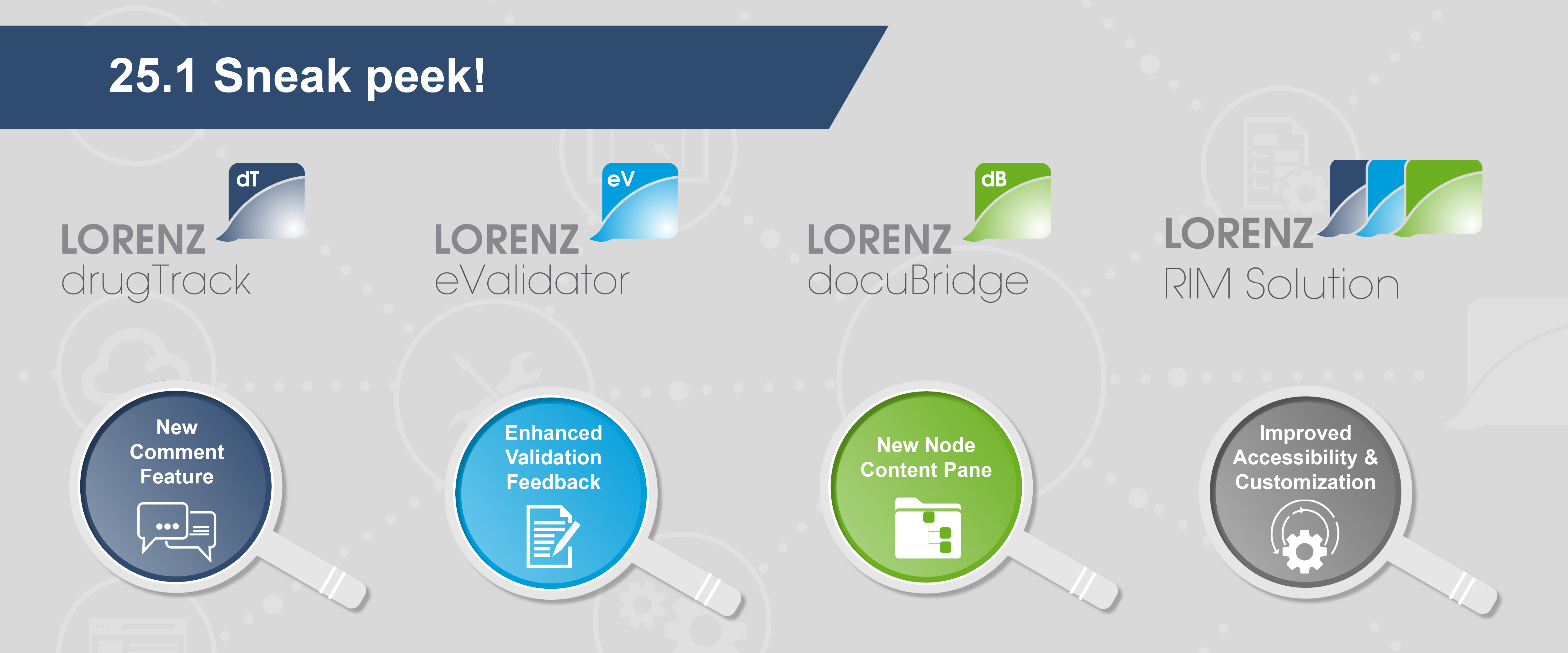
Sneak peek at the docuBridge 25.1 release:
This entry was posted in LORENZ Solutions and Services.
LORENZ Connect 2025: Global Topics around RIM, eCTD 4.0, and submissions via cloud
Posted on February 19, 2025
In the ever-evolving world of regulatory affairs, staying ahead of the curve is crucial. That’s why we are thrilled to announce LORENZ Connect 2025: our free-of-charge online conference bringing together global experts, industry leaders, and regulatory professionals for two days of insightful discussions, live demonstrations, and informative Q&A sessions. Join us on April 8 and 9, 2025, as we explore the latest trends and innovations shaping the future of regulatory affairs!

This entry was posted in LORENZ Events.
LORENZ and DocShifter – Enhancing Document Conversion Solutions
Posted on February 12, 2025
Since 2020, LORENZ has used DocShifter’s advanced technology in its cloud offerings to streamline document conversion into compliant PDFs. This collaboration has delivered fast and efficient processes, allowing customers to save time and focus on their business goals.
Building on the success of this partnership in the cloud, LORENZ is now extending the advantages of DocShifter to its on-premises customers. By integrating DocShifter as a standard feature across all LORENZ docuRender implementations, LORENZ delivers a unified and future-ready platform designed to enhance functionality for both cloud and on-premises environments.

This entry was posted in LORENZ News.
Milestones and Memories of 2024 (part4)
Posted on December 20, 2024
Welcome to the final chapter of our 2024 journey! As we reflect on an eventful year filled with milestones, memories, and meaningful connections, we are proud to share some of our most memorable moments.

This entry was posted in LORENZ News.
Milestones and Memories of 2024 (part 3)
Posted on December 16, 2024
What a year! Join us as we look back on amazing events, unforgettable moments and major successes with our customers!

This entry was posted in LORENZ News.
Milestones and Memories of 2024 (part 2)
Posted on December 9, 2024
As we approach the end of 2024, it’s time to reflect on an incredible year filled with achievements, milestones, and inspiring moments. At LORENZ, this year was defined by innovation, community, and creativity, leaving us proud of the journey we’ve shared.

This entry was posted in LORENZ News.
Milestones and Memories of 2024 (part 1)
Posted on December 3, 2024
What a year! Join us as we look back on amazing events, unforgettable moments and major successes with our customers!

This entry was posted in LORENZ News.
