Ready to enhance your efficiency with LORENZ?
Explore the world's most desirable RIM solution.
Latest News
Upcoming Events
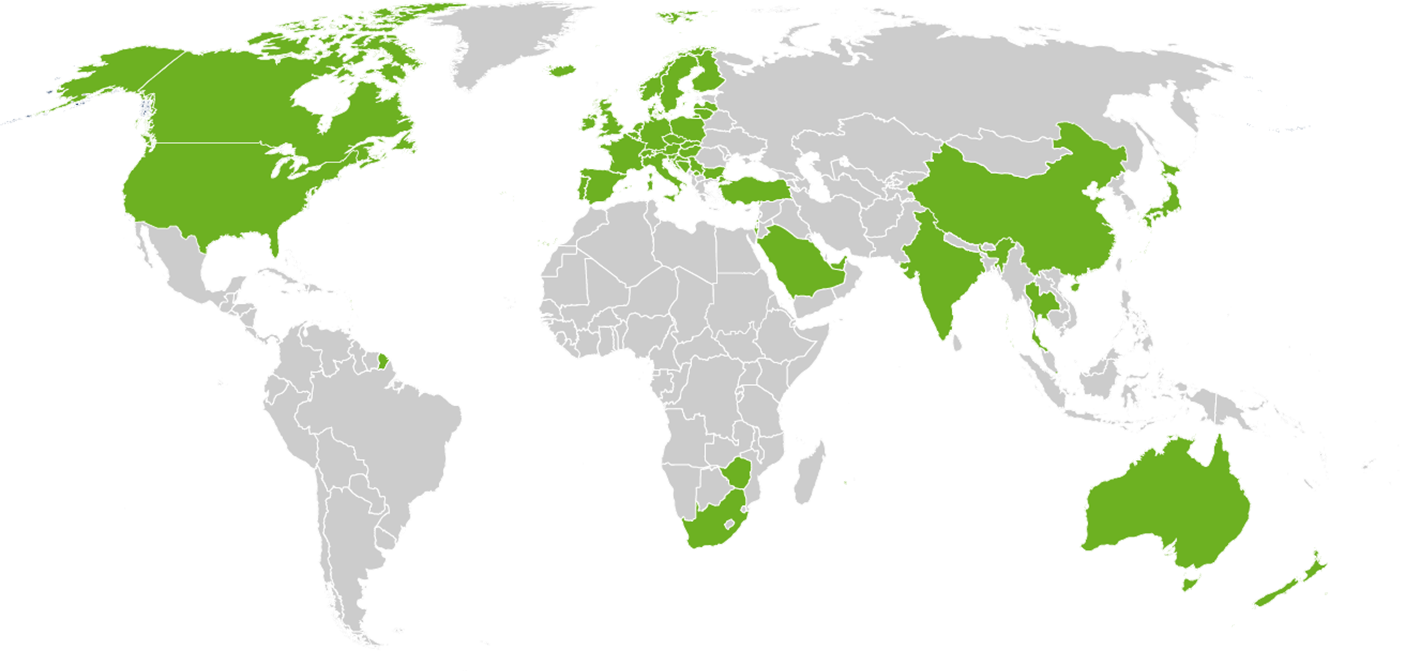
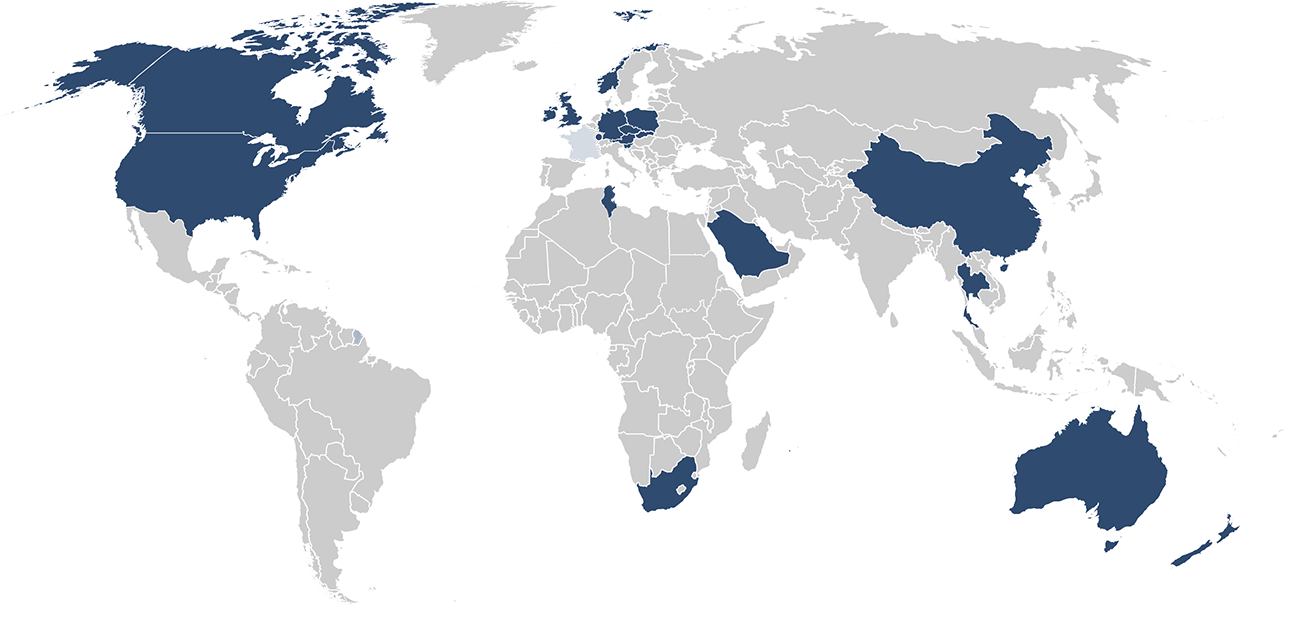
Our customers: More than 1900 paid installations in 48 countries including 19 authorities!